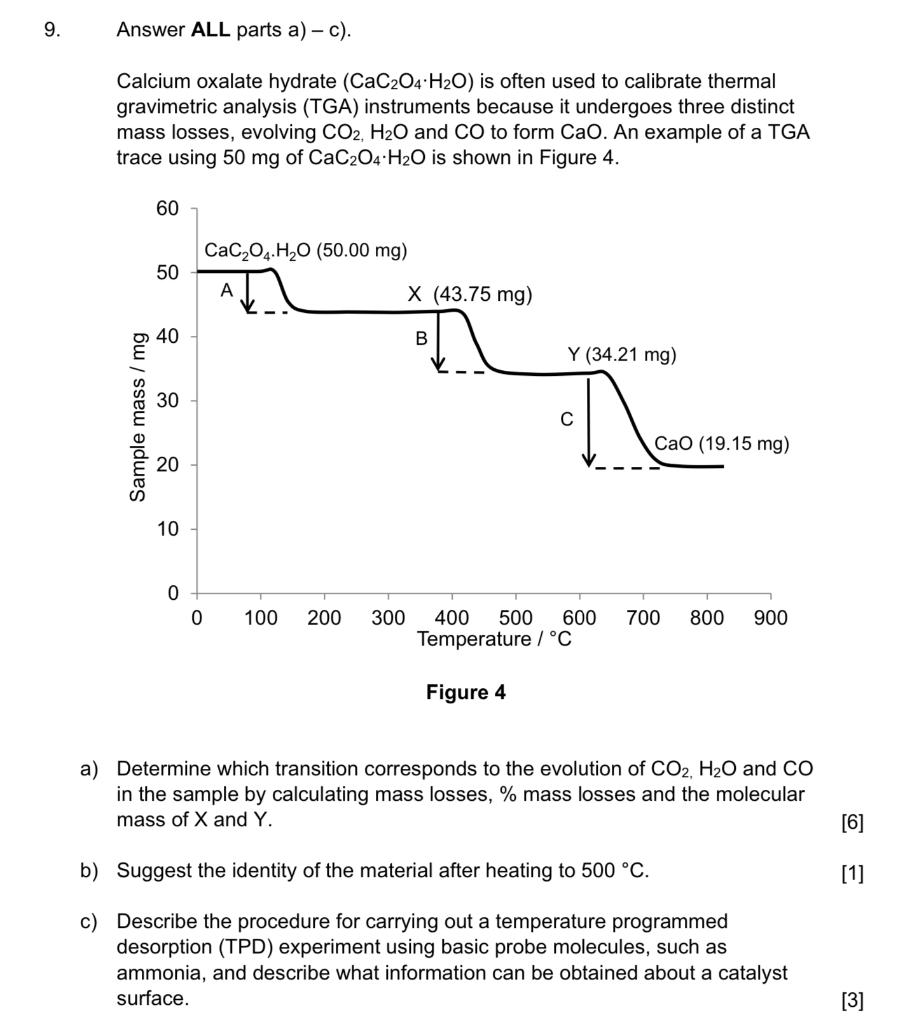
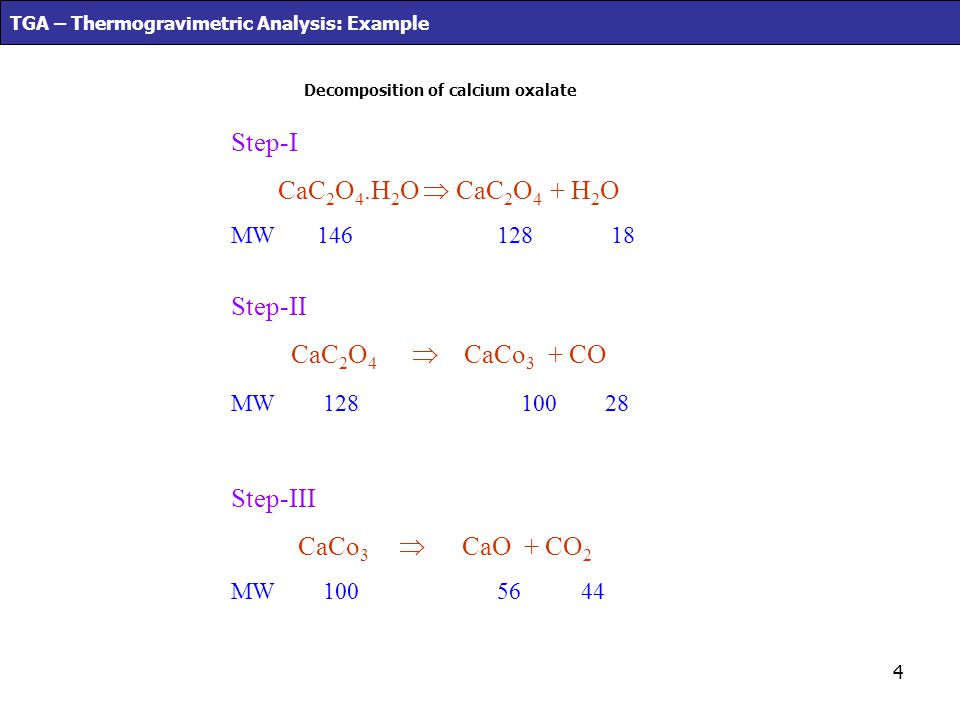
1 Chapter 3 The principle of TGA and its applications: TGA is thermogravimetric analysis. It is one of the thermal method of an
1 Chapter 3 The principle of TGA and its applications: TGA is thermogravimetric analysis. It is one of the thermal method of an
Whewellite, CaC2O4⋅H2O: structural study by a combined NMR, crystallography and modelling approach - CrystEngComm (RSC Publishing)

SOLVED: Consider a saturated solution of calcium oxalate, CaC2O4, in which the following equilibrium can occur: CaC2O4(s) ⇌ Ca2+ (aq) + C2O42- (aq) Ksp = 1.3 × 10-8 C2O42- (aq) + H2O (

Lab #2 Gravimetric Determination of Calcium as CaC2O4·H2O.pptx - Gravimetric Determination of Calcium as CaC2O4H2O Chem 523L Lab #2 What is | Course Hero
SOLVED: What is the concentration of calcium (Ca2+) in calcium oxalate monohydrate (CaC2O4.H2O). Ca2+(aq) + C2O42-(aq) + H2O (l) → CaC2O4·H2O (s) The mass of calcium oxalate monohydrate is 0.190grams. what is