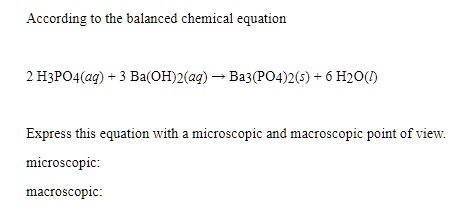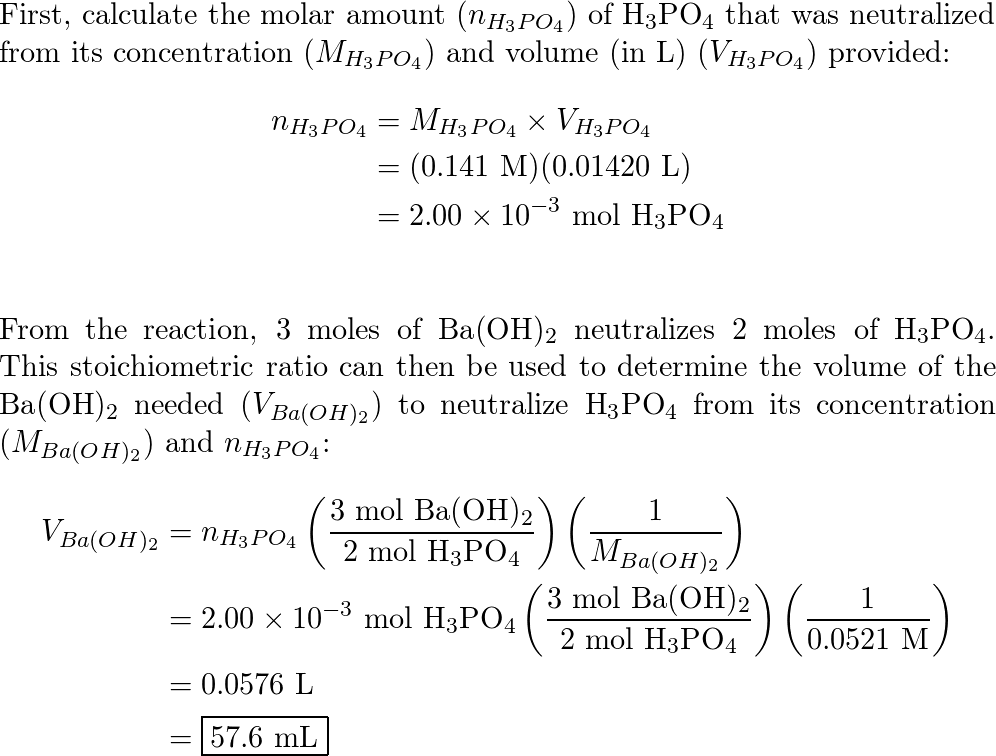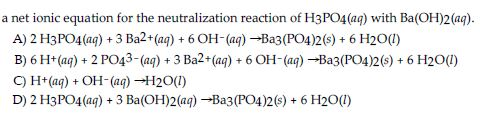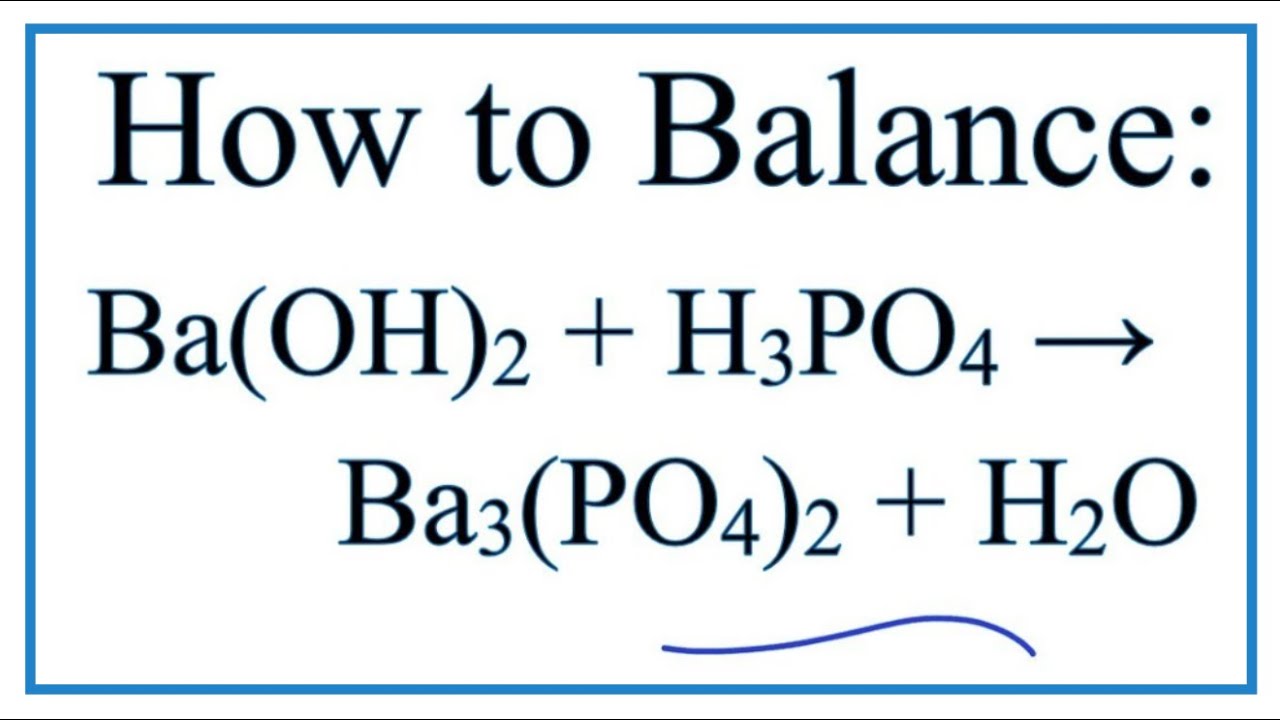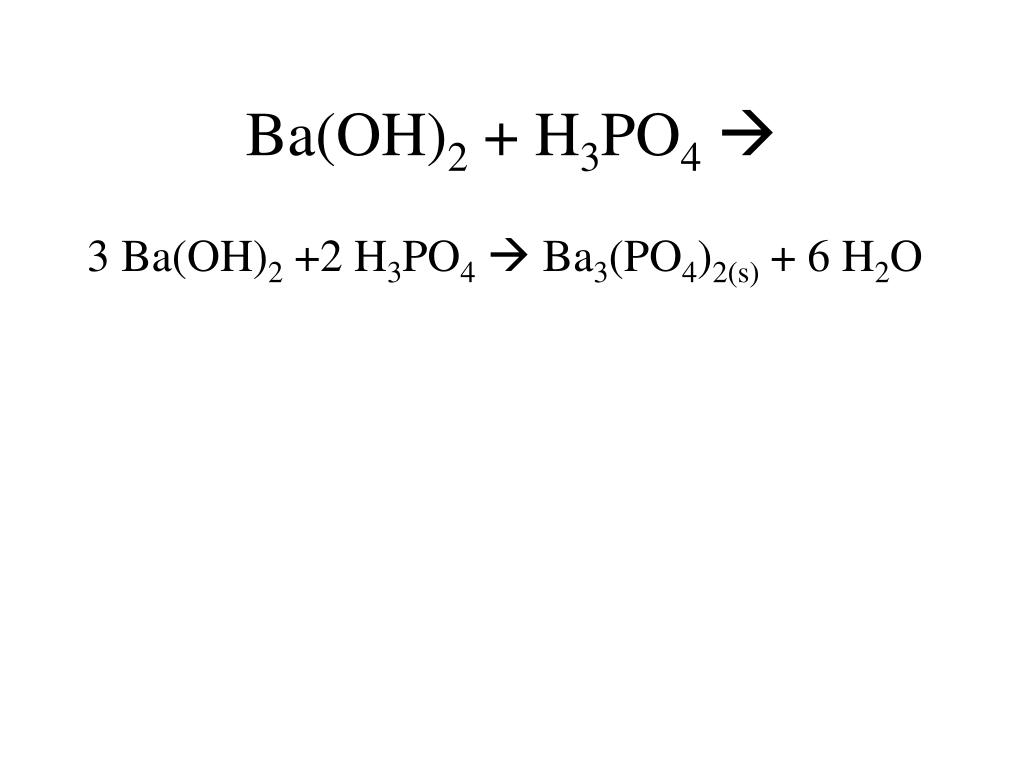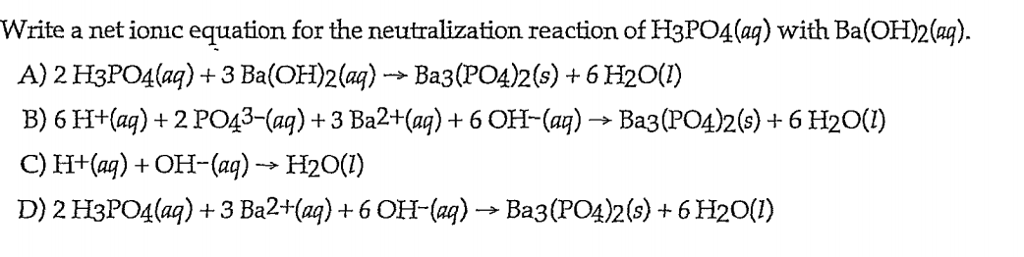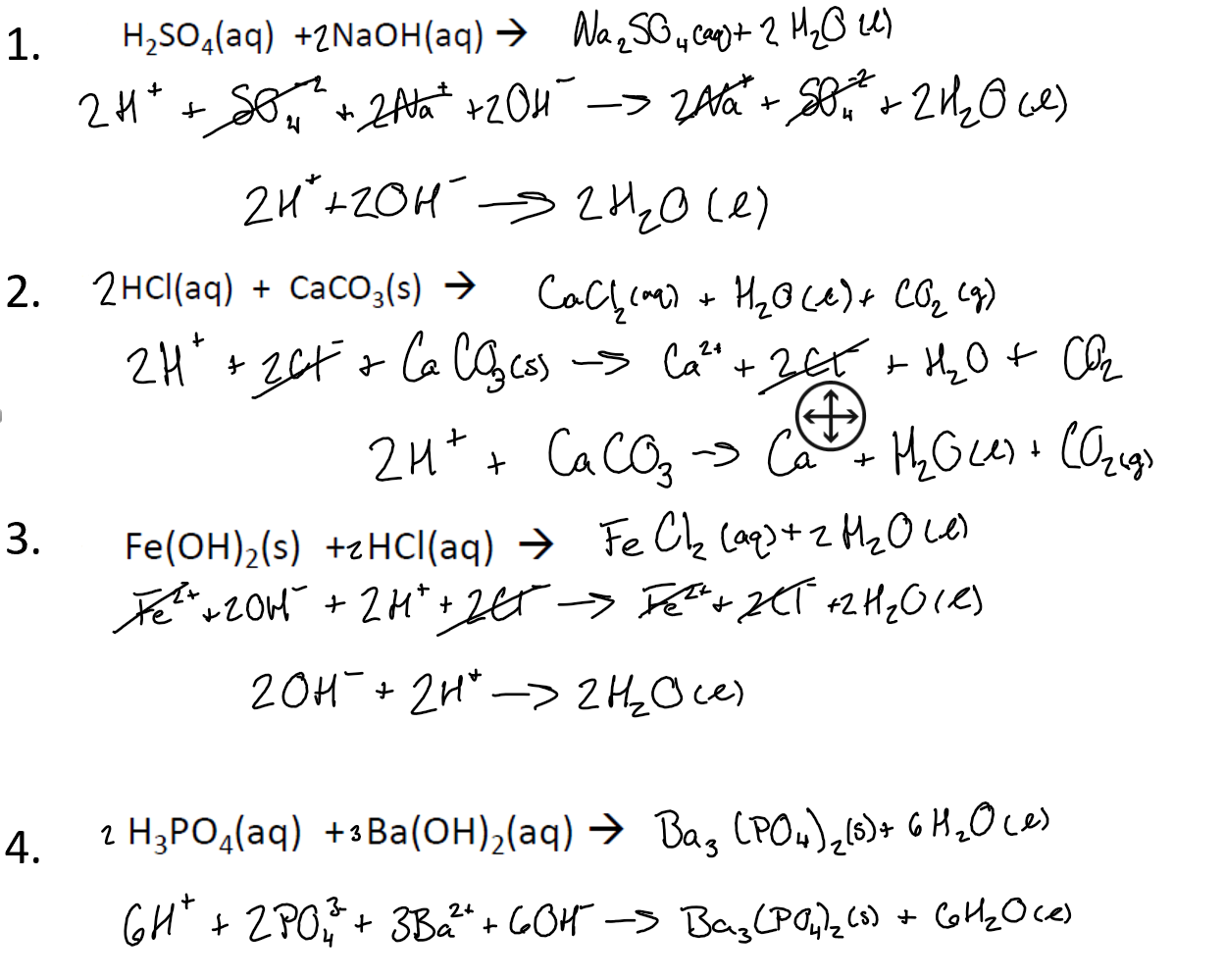
SOLVED: What would be the total ionic equation for 3Ba(OH)2 (aq) + 2H3PO4 (aq) → Ba3(PO4)2 (s) + 6H2O (aq)? Identify the spectator ions of this reaction.
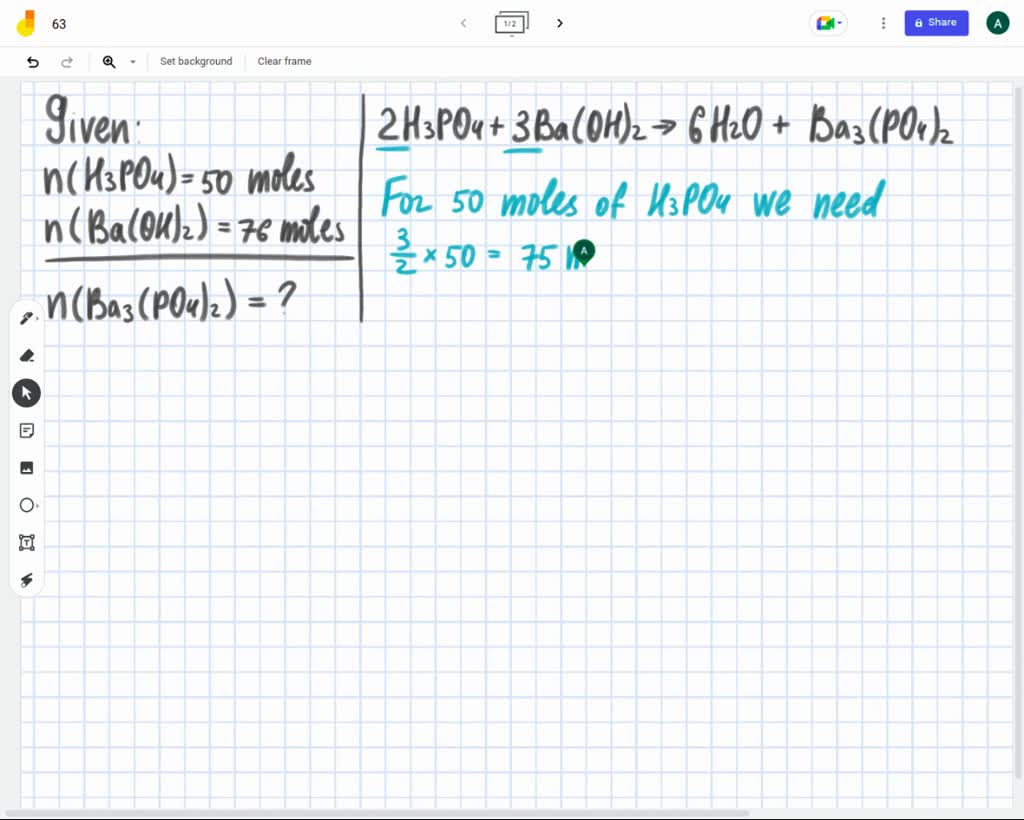
SOLVED: Given the balanced reaction, 2 H3PO4(aq)+ 3 Ba(OH)2(aq)> 6 H2O(l)+ Ba3(PO4)2(s), how many moles of Ba3(PO4)2(s) will be produced from a reaction mixture composed of 50 moles of H3PO4and 76 moles

SOLVED: If 30.0 mL of 0.15 M Ba(OH)2 was needed to neutralize 50.0 mL of an H3PO4solution. What is the concentration of the original H3PO4 solution?
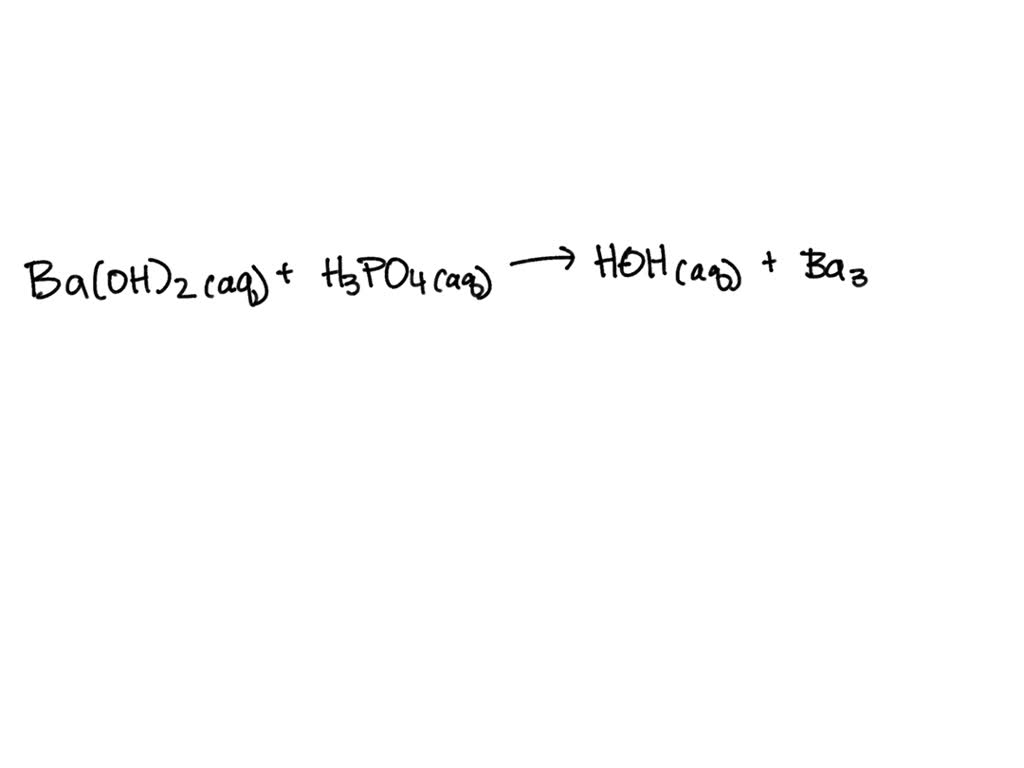
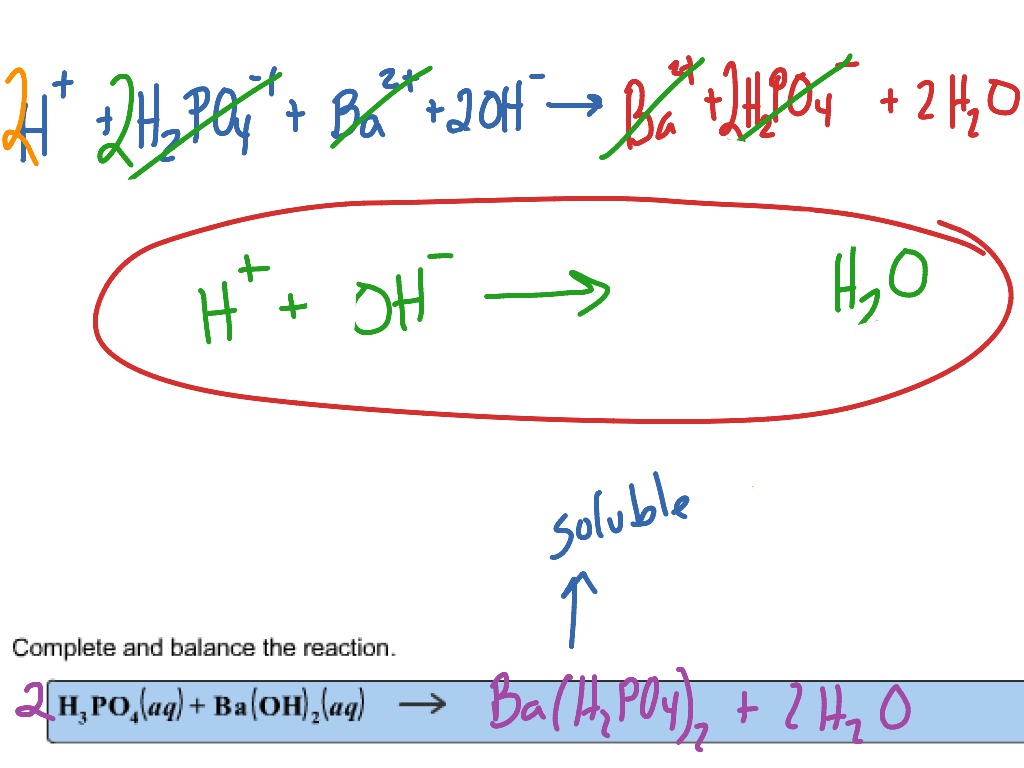
SOLVED: When a solution containing barium hydroxide is added to a solution containing phosphoric acid (HaPO4), a white precipitate forms and settles to the bottom of the beaker: What is the white

SOLVED: Aqueous solutions of barium hydroxide (Ba(OH)2) and phosphoric acid (H3PO4) will react to yield barium phosphate and water. In the balanced equation for this reaction, what is the lowest possible whole