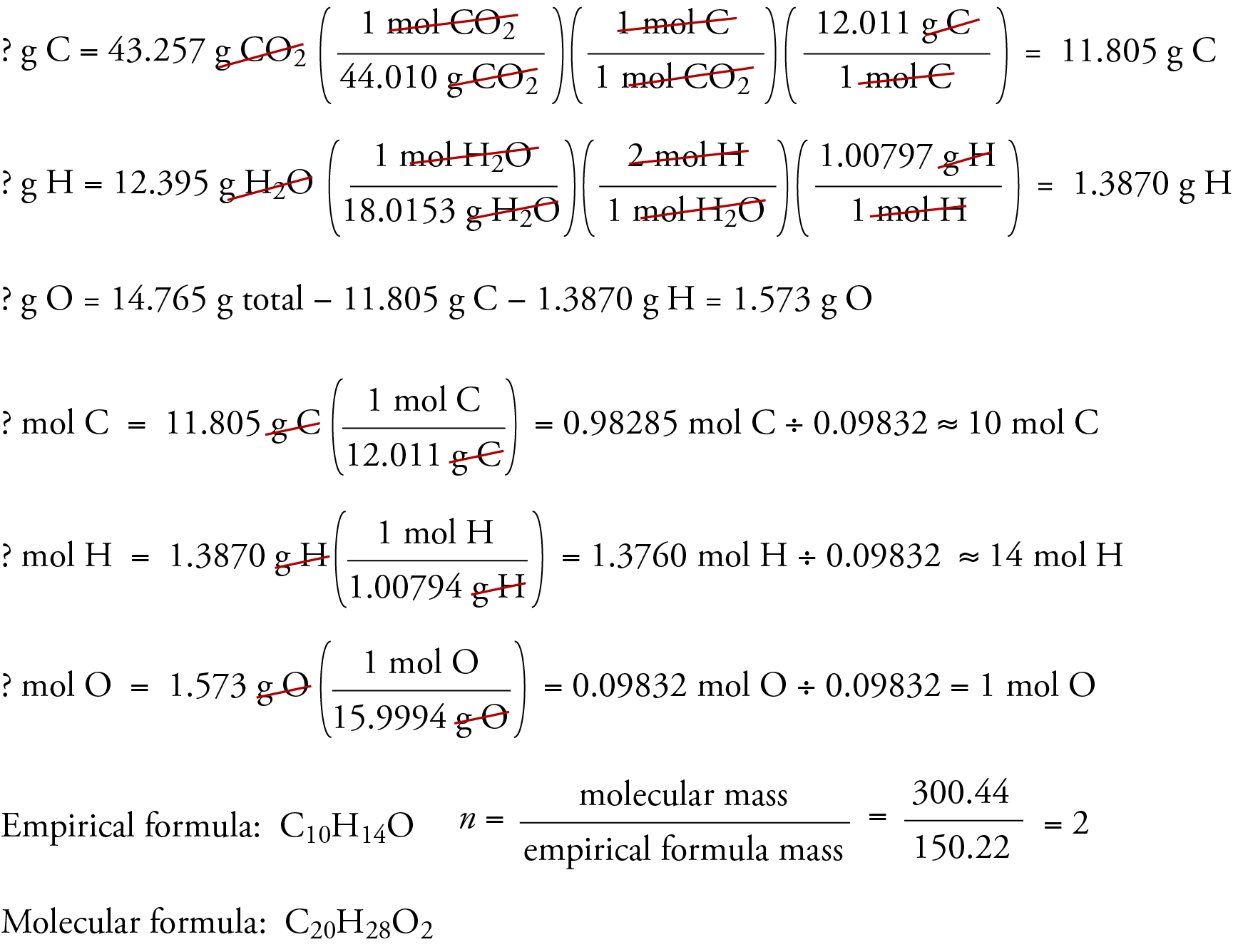
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are

Grundlegende operationen der farbenchemie . gefrührt: Man löst 1 Molekül des Pnoles in 400 CMS Wasser,1 mol. Natronlauge et Soda, 80 g. Zu dieser Lösung gibtman 500 cm^ Alkohol (Methyl- bezw.

Struers MD-Mol Poliertuch Ø 300 mm 40500079 | Amikon-Shop.de | An - und Verkauf von gebrauchter Industrieelektonik
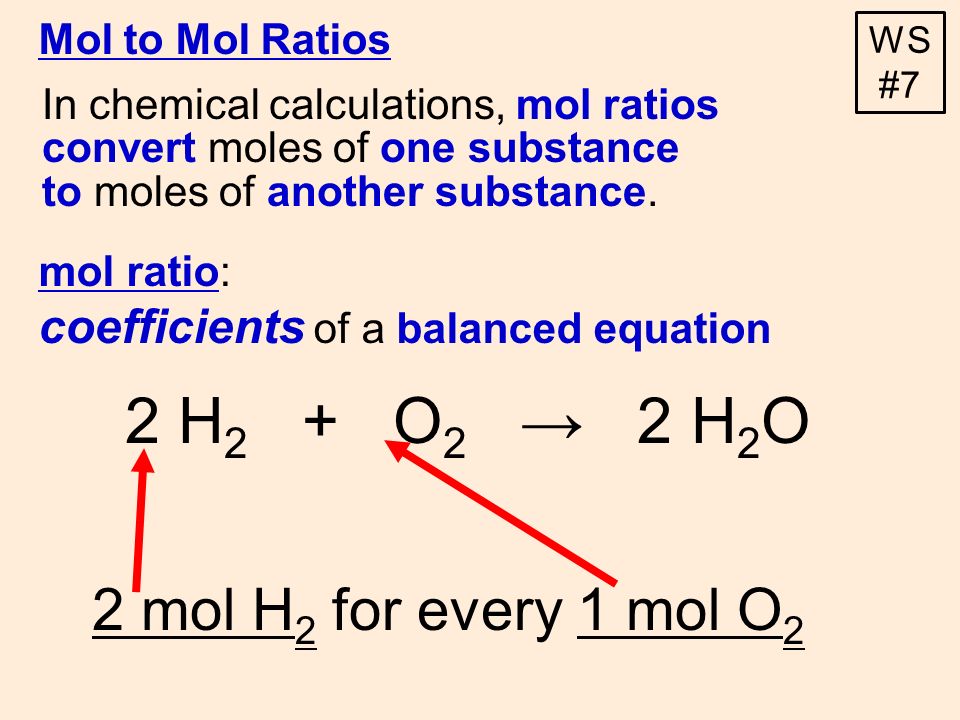
Mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O 2 mol H 2 for every 1 mol O 2 In chemical calculations, mol ratios convert moles of. - ppt download

Chemistry Warm Up: Mole / Mass / Particles 1.What is the mass of one mole of water? 2.If one milliliter of water has a mass of 1.00grams, how many moles. - ppt download

SOLVED: Determine the number of moles of oxygen atoms in each sample. a. 4.88 mol H2O2 b. 2.15 mol N2O c. 0.0237 mol H2CO3 d. 24.1 mol CO2

Question Video: Calculating the Mass of Solute Needed to Prepare a Solution with a Desired Concentration and Volume | Nagwa

O−H Bond Dissociation Enthalpies in Oximes: Order Restored | Journal of the American Chemical Society

Mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O 2 mol H 2 for every 1 mol O 2 In chemical calculations, mol ratios convert moles of. - ppt download

Practice Problem How many moles of aluminum oxide will be produced from 0.50 mol of oxygen? 4 Al + 3 O 2 → 2 Al 2 O mol? mol 3 O 2 = 2 Al 2 O ppt download