![Sodium Borate Decahydrate [Na2B4O7.10H2O] 99.9% ACS Grade Powder 1 Lb in Two Space-Saver Bottles USA: Amazon.com: Industrial & Scientific Sodium Borate Decahydrate [Na2B4O7.10H2O] 99.9% ACS Grade Powder 1 Lb in Two Space-Saver Bottles USA: Amazon.com: Industrial & Scientific](https://m.media-amazon.com/images/I/91oK0hZSWxL._AC_UF1000,1000_QL80_.jpg)
Sodium Borate Decahydrate [Na2B4O7.10H2O] 99.9% ACS Grade Powder 1 Lb in Two Space-Saver Bottles USA: Amazon.com: Industrial & Scientific

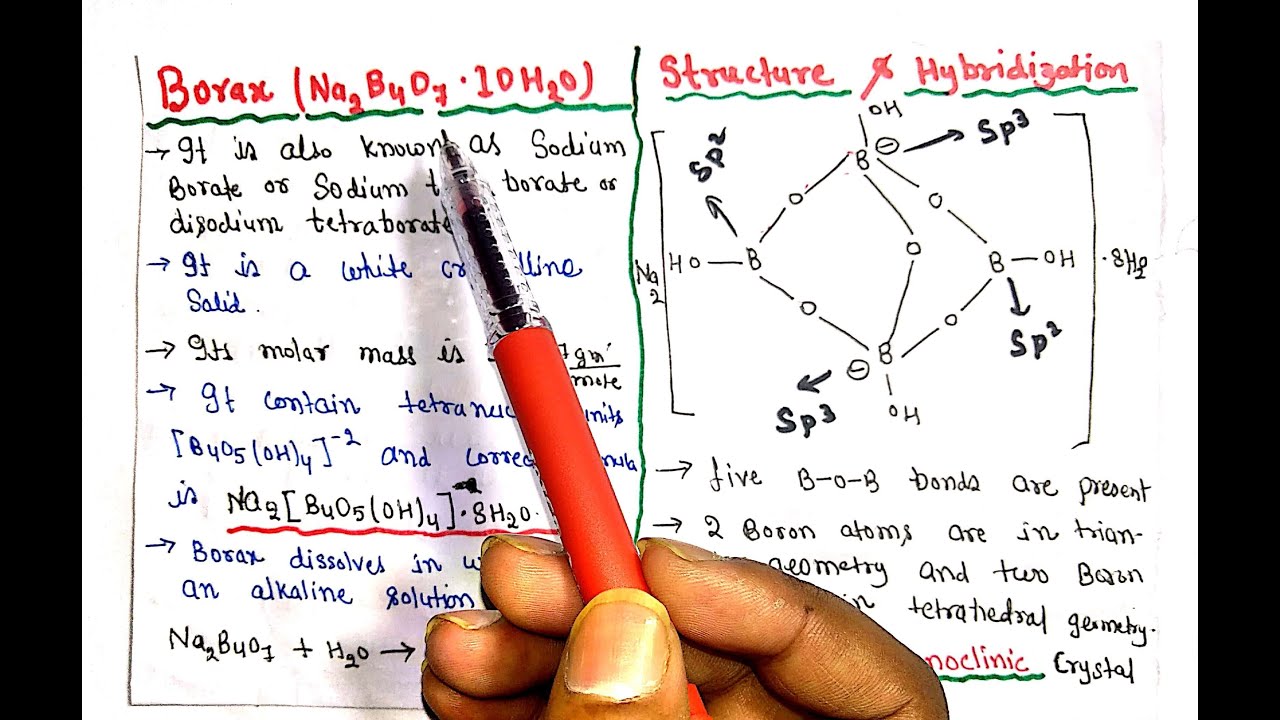
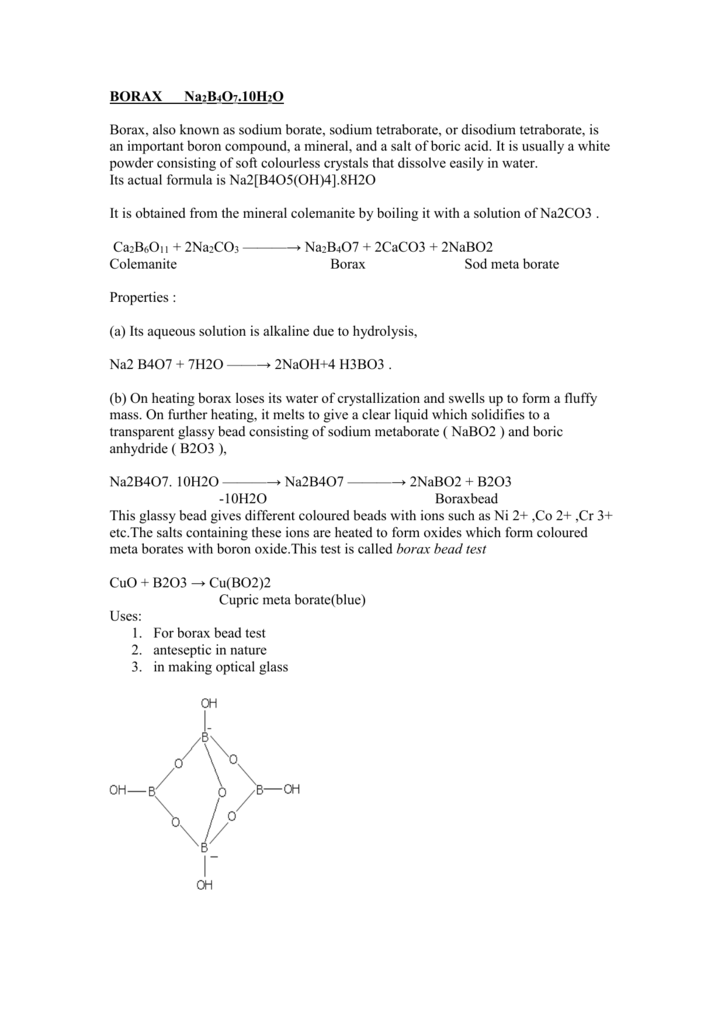
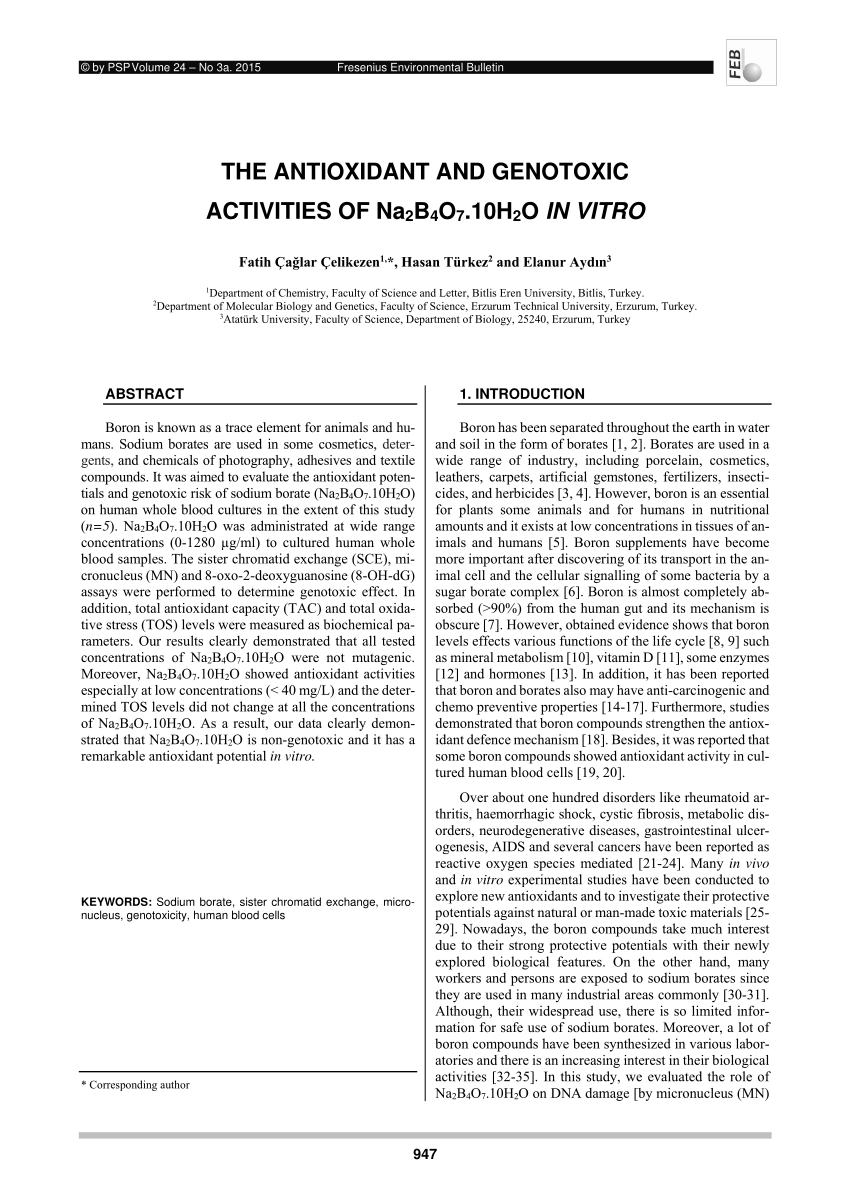
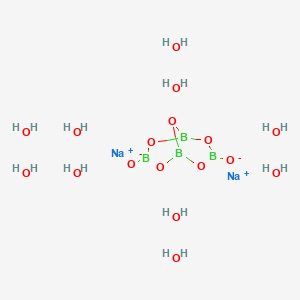
Borax has the formula Na2B4O7. 10H2O. It is a strong base in aqueous solution because OH^- ions are produced by reaction with water. (B4O7^2 - + 7H2O → 4H3BO3 + 2OH^-). How
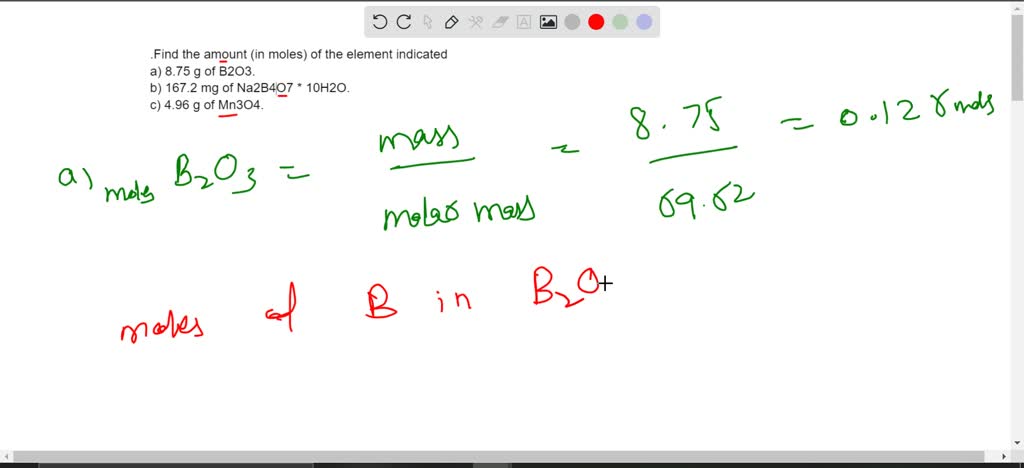
SOLVED: 1.Find the amount (in moles) of the element indicated a) 8.75 g of B2O3. b) 167.2 mg of Na2B4O7 * 10H2O. c) 4.96 g of Mn3O4.2. What is the mass in
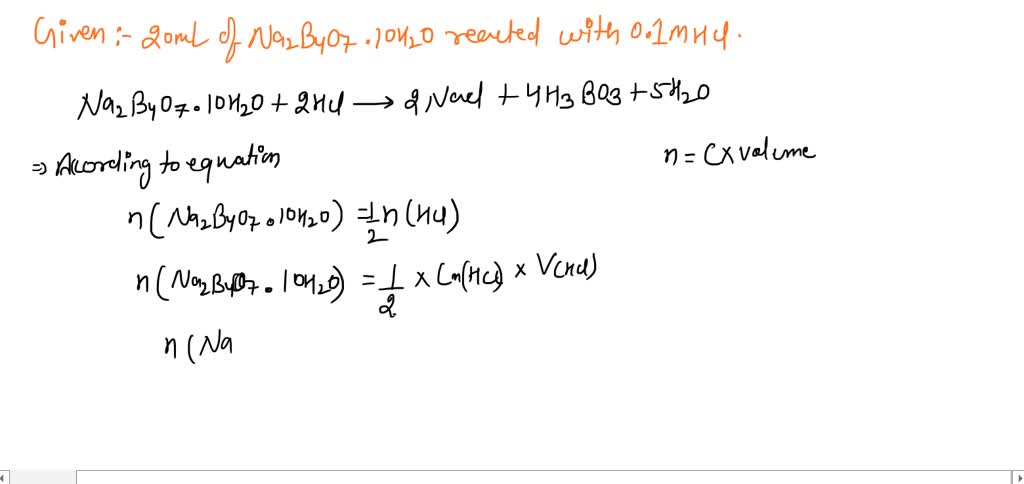
SOLVED: Calculate the mass of borax (sodium tetraborate decahydrate, Na2B4O7 .10H2O) required to give a titre volume of 20.00 mL when reacted with 20.00 mL of 0.1 mol L–1 HCl. Show your working.
![Sodium Borate Decahydrate [Na2B4O7.10H2O] 99.9% ACS Grade Powder 1 Lb in Two Space-Saver Bottles USA: Amazon.com: Industrial & Scientific Sodium Borate Decahydrate [Na2B4O7.10H2O] 99.9% ACS Grade Powder 1 Lb in Two Space-Saver Bottles USA: Amazon.com: Industrial & Scientific](https://m.media-amazon.com/images/I/91oK0hZSWxL.jpg)
Sodium Borate Decahydrate [Na2B4O7.10H2O] 99.9% ACS Grade Powder 1 Lb in Two Space-Saver Bottles USA: Amazon.com: Industrial & Scientific




![Borax Decahydrate 10 Mol [Na2B4O7.10H2O] [CAS_1303-96-4] Technical Sta – Wintersun Chemical Borax Decahydrate 10 Mol [Na2B4O7.10H2O] [CAS_1303-96-4] Technical Sta – Wintersun Chemical](https://cdn.shopify.com/s/files/1/0724/7981/products/02-010-1.jpg?v=1532727515)
