Complications from dual roles of sodium hydride as a base and as a reducing agent. | Semantic Scholar
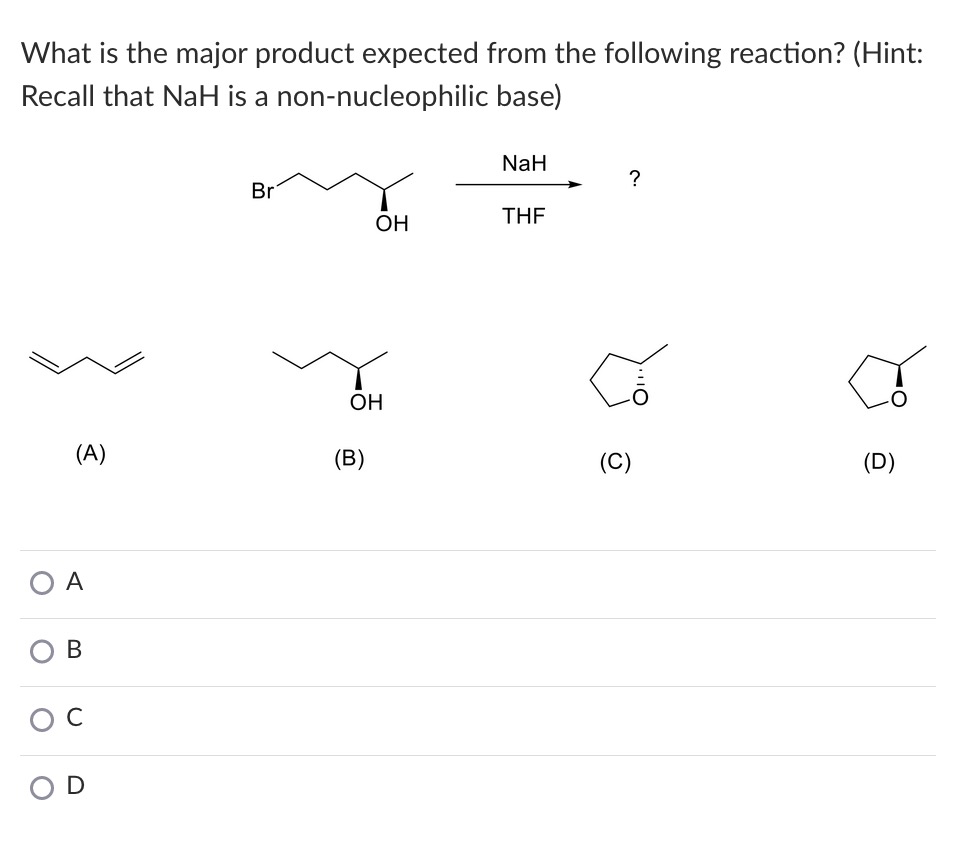
✓ Solved: When 4-chlorobutane-1-thiol is treated with a strong base such as sodium hydride, NaH, tetrahydrothiophene...
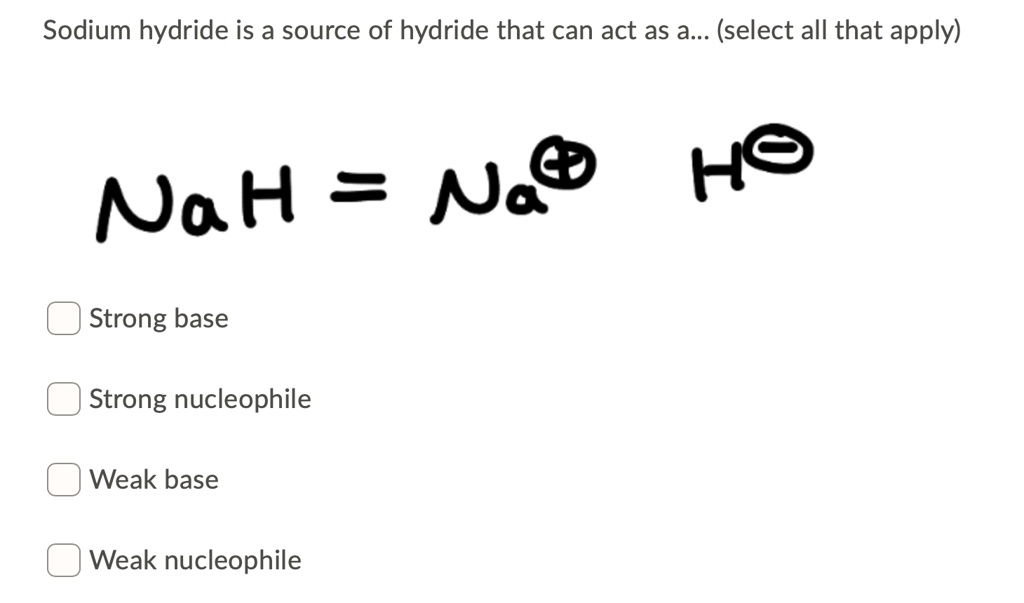
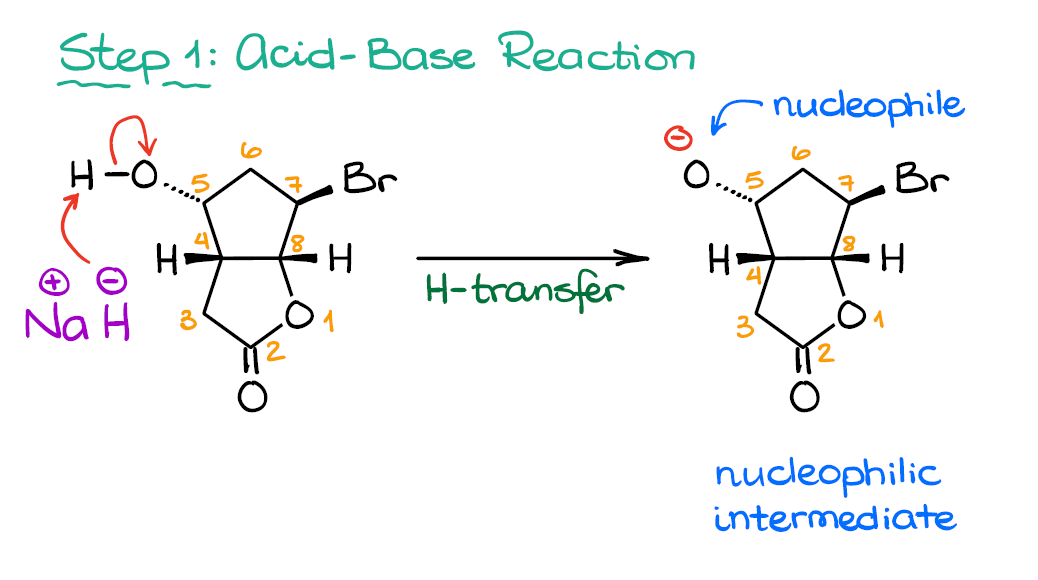
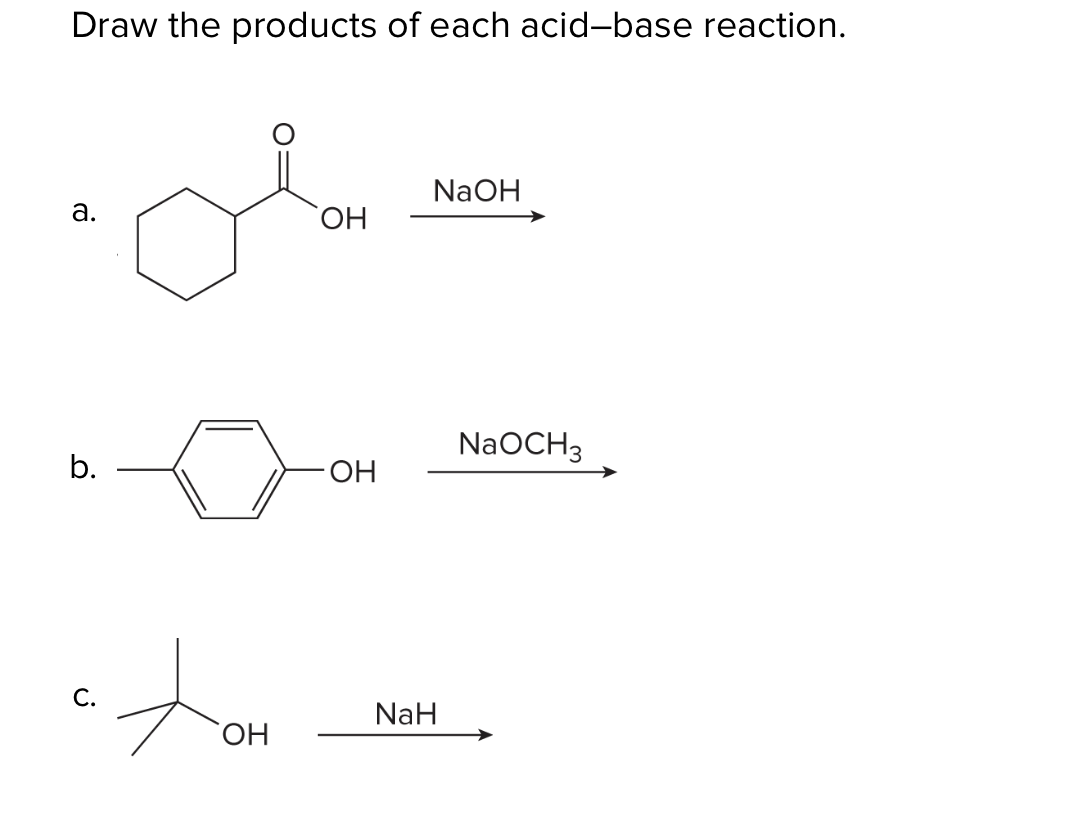
SOLVED: Sodium hydride is a source of hydride that can act as a (select all that apply) Na@ HO NaH = Strong base Strong nucleophile Weak base Weak nucleophile

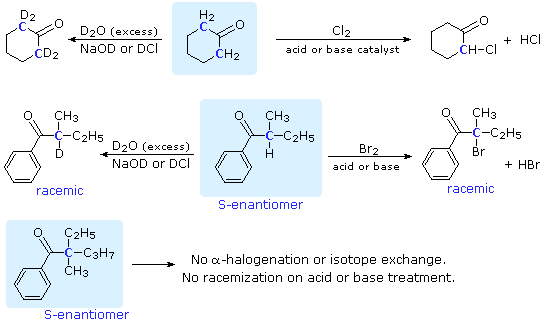
Commonly Used Hydride Reagents. Several forms of hydride (H-) find use in organic chemistry, including NaH, CaH 2, LiAlH 4, NaBH 4, and NaBH 3 CN (and. - ppt download

I'm having trouble determining what the base in this E1 reaction is , anyone has an idea ? : r/OrganicChemistry

The hydride ion, H^- is a stronger base than its hydroxide ion OH^- . Which of the following reactions will occur, if sodium hydride (NaH) is dissolved in water?