The system Al2O3-P2O5-H2O at temperatures below 200 °C: Experimental data on the stability of variscite and metavariscite AlPO4·2H2O | Semantic Scholar

PDF) Heat capacity and glass transition in P2O5–H2O solutions: support for Mishima's conjecture on solvent water at low temperature | Federico Nores Pondal - Academia.edu
![PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e9e458615e33c40d94b80a3189efa26d438c8a39/3-Table1-1.png)
PDF] Heat capacity and glass transition in P2O5-H2O solutions: support for Mishima's conjecture on solvent water at low temperature. | Semantic Scholar
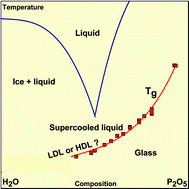
Heat capacity and glass transition in P2O5–H2O solutions: support for Mishima's conjecture on solvent water at low temperature - Physical Chemistry Chemical Physics (RSC Publishing)

one mole of p2o5 undergoes hydrolysis as P2O5 + H2O ----- H3PO4 WHAT IS THE NORMALITY OF PHOSPHORIC - Chemistry - Some Basic Concepts of Chemistry - 4612005 | Meritnation.com

CCLXXXI.—The systems B2O3–SO3–H2O and B2O3–P2O5–H2O - Journal of the Chemical Society (Resumed) (RSC Publishing)

PH3+O2=P2O5+H2O нужно разобрать как ОВР(окислительно-восстановительная реакция) - Школьные Знания.com
Complete and balance the following equation (i) P2O5 + H2O → ............ (ii) S + O2 → ................ - Sarthaks eConnect | Largest Online Education Community