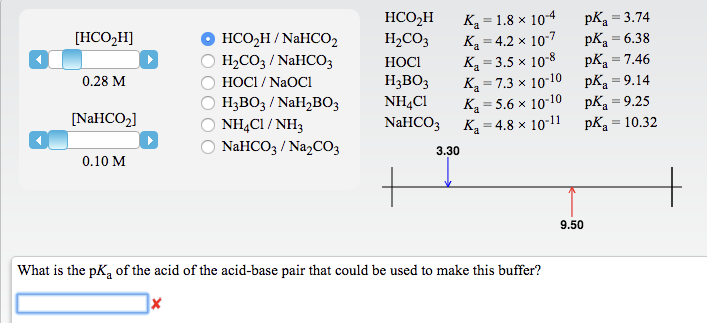
physical chemistry - Which make HCO3- to show two pH values at two scenarios? - Chemistry Stack Exchange
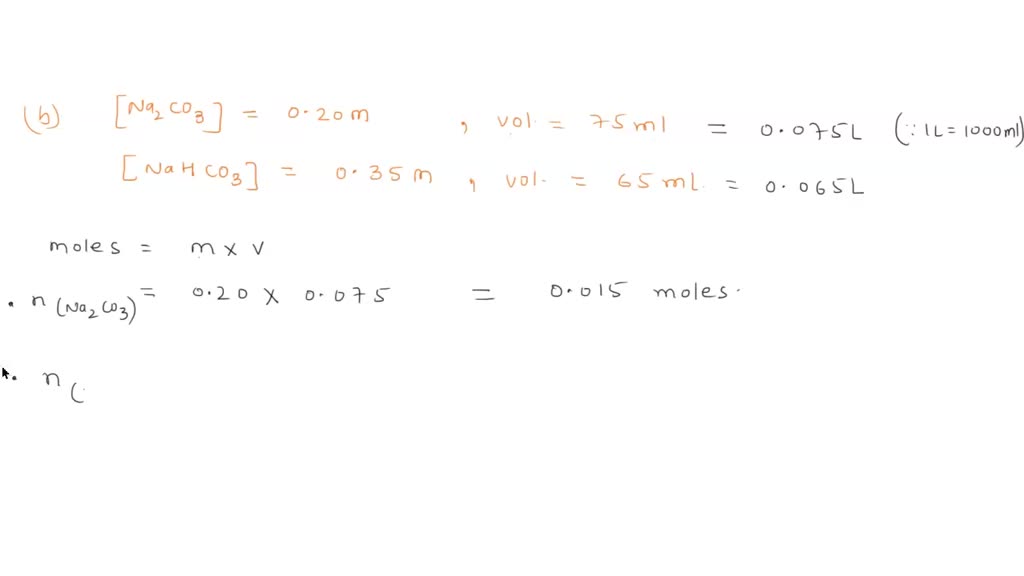
SOLVED: Consider a buffer solution that contains 0.35 M NaHCO3 and 0.25 M Na2CO3. pKa(HCO3-)=10.33. If the acceptable buffer range of the solution is ±0.10 pH units, calculate how many moles of
![SOLVED: A buffer made from NaHCO3 and Na2CO3 is prepared with a pH of 9.40. a. What must the [CO3 ] /[HCO3 ] ratio be? Ka for HCO3 is 4.7 x 10 . SOLVED: A buffer made from NaHCO3 and Na2CO3 is prepared with a pH of 9.40. a. What must the [CO3 ] /[HCO3 ] ratio be? Ka for HCO3 is 4.7 x 10 .](https://cdn.numerade.com/previews/bd62afea-5060-4429-b9f4-4515705d9462_large.jpg)
SOLVED: A buffer made from NaHCO3 and Na2CO3 is prepared with a pH of 9.40. a. What must the [CO3 ] /[HCO3 ] ratio be? Ka for HCO3 is 4.7 x 10 .

Determination of acid/base dissociation constants based on a rapid detection of the half equivalence point by feedback-based flow ratiometry. | Semantic Scholar

Development of a Robust Protocol for the Determination of Weak Acids' pKa Values in DMSO | The Journal of Organic Chemistry
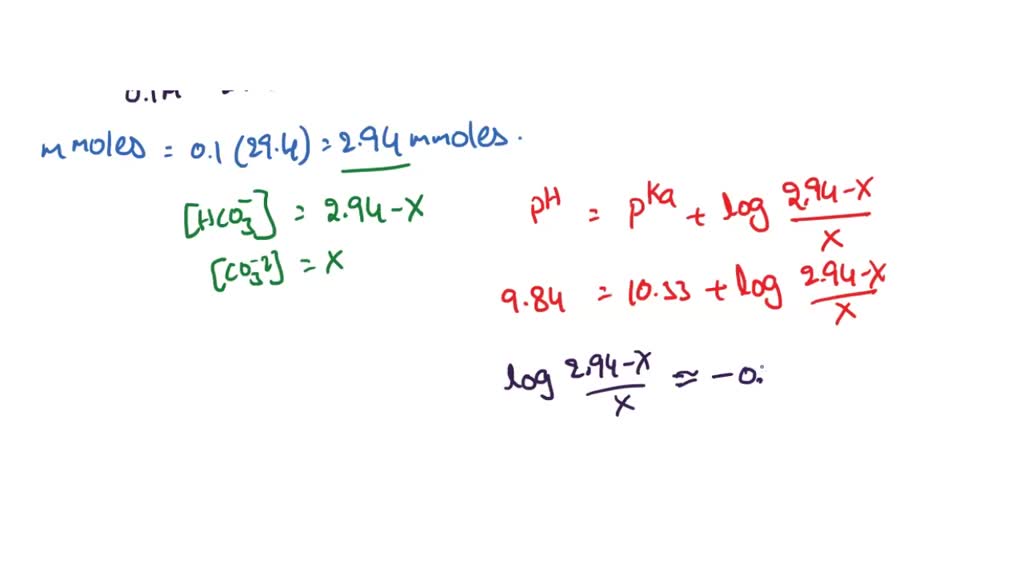
SOLVED: You are asked to prepare 500 mL of NaHCO3/Na2CO3 buffer of pH 9.87. What is the mole ratio for Na2CO3 and NaHCO3 that you are goanna mix up? For the ionization



![The pKa values for various precipitants [17]. | Download Scientific Diagram The pKa values for various precipitants [17]. | Download Scientific Diagram](https://www.researchgate.net/publication/339359335/figure/tbl1/AS:860297669640196@1582122356901/The-pKa-values-for-various-precipitants-17_Q320.jpg)