SOLVED: What is the mass in grams of each of the following samples? (a) 1.505 mol of Ti (b) 0.337 mol of Na (c) 2.583 mol of U
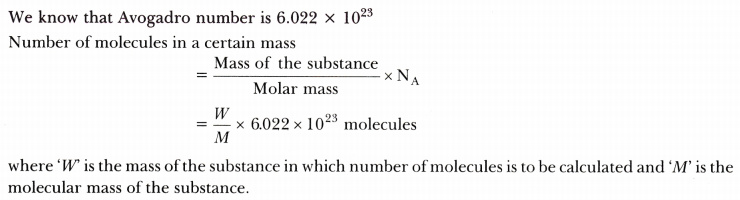
What is a mole? What is the unit of mole? How many molecules are there in a certain mass of a substance? - CBSE Class 9 Science - Learn CBSE Forum
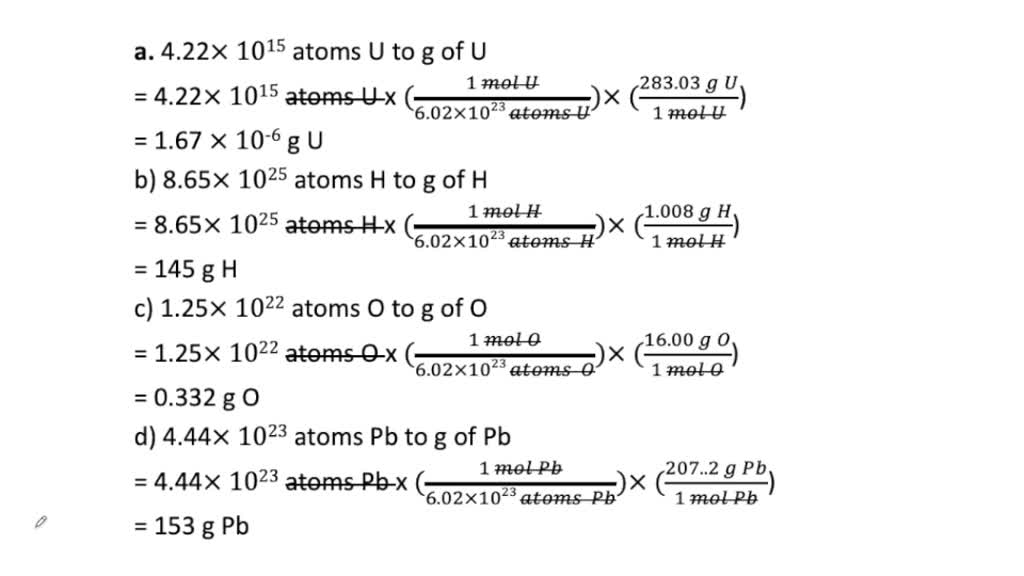
SOLVED:Convert each to mass in grams. a. 4.22 ×10^15 atoms U b. 8.65 ×10^25 atoms H c. 1.25 ×10^22 atoms O d. 4.44 ×10^23 atoms Pb
The unit of rate constant of a reaction is ("mol")^(-x).(dm)^(3x).s^(-1) Find the value of x if the order of the reaction is (3)/(2) .

Chem Notes Mole- SI unit for amount of matter Mole- SI unit for amount of matter mol 6.02 X representative particles= Avogadro's Number. - ppt download